"Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and 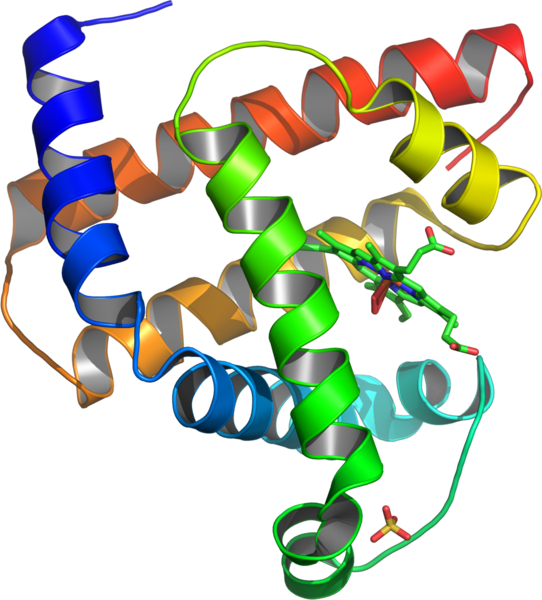 in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the bloodstream is when it is released following muscle injury. It is an abnormal finding, and can be diagnostically relevant when found in blood. [2]"
in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the bloodstream is when it is released following muscle injury. It is an abnormal finding, and can be diagnostically relevant when found in blood. [2]"
"Myoglobin (abbreviated Mb) is a single-chain globular protein of 153[3] or 154[4] amino acids, containing a heme (iron-containing porphyrin) prosthetic group in the center around which the remaining apoprotein folds. It has eight alpha helices and a hydrophobic core. It has a molecular weight of 17,699 (with heme)daltons, and is the primary oxygen-carrying pigment of muscle tissues.[4] Unlike the blood-borne hemoglobin, to which it is structurally related,[5] this protein does not exhibit cooperative binding of oxygen, since positive cooperativity is a property of multimeric/oligomeric proteins only. Instead, the binding of oxygen by myoglobin is unaffected by the oxygen pressure in the surrounding tissue. Myoglobin is often cited as having an "instant binding tenacity" to oxygen given its hyperbolic oxygen dissociation curve. High concentrations of myoglobin in muscle cells allow organisms to hold their breaths longer. Diving mammals such as whales and seals have muscles with particularly high myoglobin abundance.[2]"