"When it's pure, hydrogen peroxide is an almost colourless (very pale blue) substance that resembles water 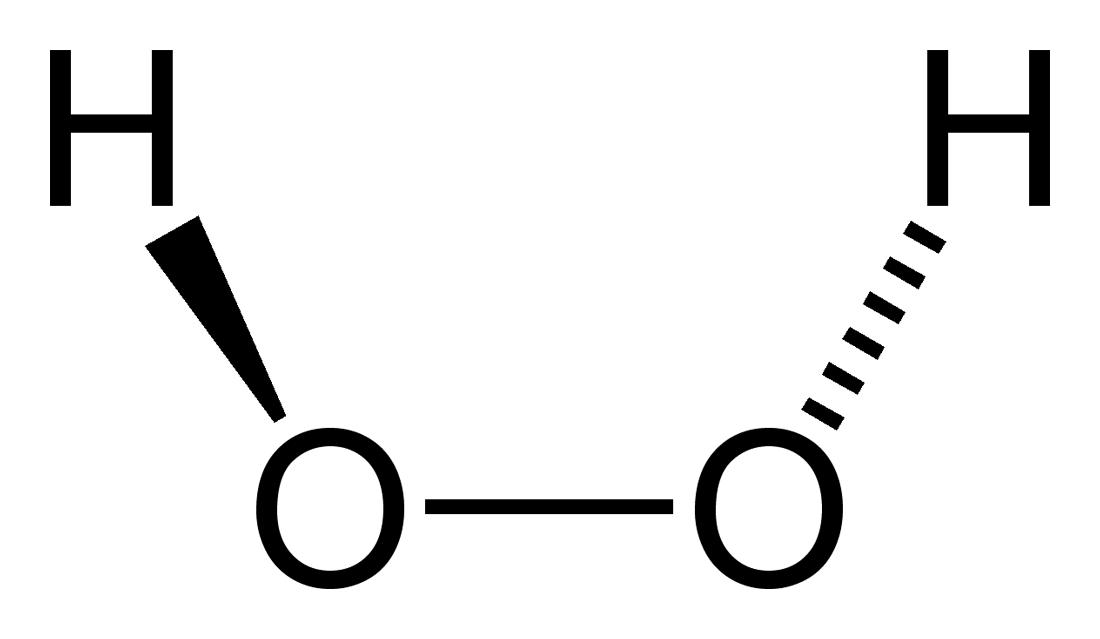 (and mixes with it in all proportions). It freezes at -0.41°C and boils at 150.2°C (extrapolated to 760 mm pressure). Its density is 1.44 g cm-3 in the liquid state at 25°C and 1.64 g cm-3 in the solid state at -4.5°C, so that, unlike ice, solid H2O2 sinks when placed in the corresponding liquid form. It has a skewed structure with a dihedral angle of 111.5° (gas phase), which minimises repulsion between the lone pairs and the O-H bond pairs. The dihedral angle is affected by hydrogen bonding; it is 90.2° in solid H2O2."
(and mixes with it in all proportions). It freezes at -0.41°C and boils at 150.2°C (extrapolated to 760 mm pressure). Its density is 1.44 g cm-3 in the liquid state at 25°C and 1.64 g cm-3 in the solid state at -4.5°C, so that, unlike ice, solid H2O2 sinks when placed in the corresponding liquid form. It has a skewed structure with a dihedral angle of 111.5° (gas phase), which minimises repulsion between the lone pairs and the O-H bond pairs. The dihedral angle is affected by hydrogen bonding; it is 90.2° in solid H2O2."