"Dioxin, the short name for 2,3,7,8-tetrachlorodibenzo-para-dioxin (2,3,7,8-TCDD), is considered to be one of 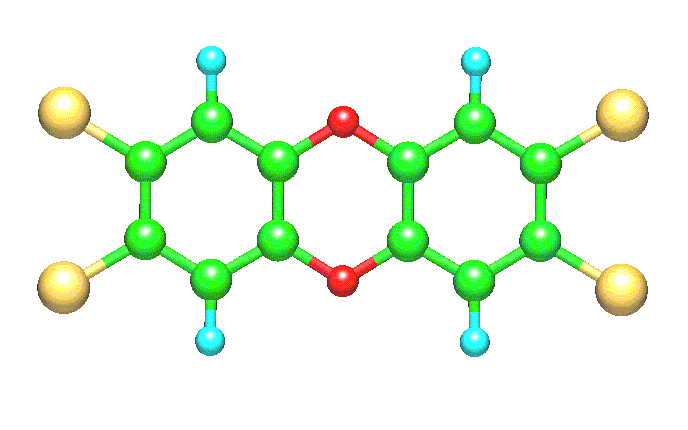 the most dangerous compounds that pollute our environment. Its chemical structure is made of two aromatic rings joined through a pair of oxygen atoms, as shown in the above drawing. Four chlorine atoms, two on each aromatic ring, are attached at positions 2, 3, 7, and 8. The whole molecule is flat-like and possesses D2h symmetry. If we consider that up to eight chlorine atoms can be attached to the dibenzodioxin (DD) skeleton, then 75 chlorine-substituted DD isomers can be conceived. Dioxin is inextricably linked to environmental pollution from waste incineration and to its incidental formation in chemical plants that are devoted to the production of pesticides [Tuppurainen 2003]."
the most dangerous compounds that pollute our environment. Its chemical structure is made of two aromatic rings joined through a pair of oxygen atoms, as shown in the above drawing. Four chlorine atoms, two on each aromatic ring, are attached at positions 2, 3, 7, and 8. The whole molecule is flat-like and possesses D2h symmetry. If we consider that up to eight chlorine atoms can be attached to the dibenzodioxin (DD) skeleton, then 75 chlorine-substituted DD isomers can be conceived. Dioxin is inextricably linked to environmental pollution from waste incineration and to its incidental formation in chemical plants that are devoted to the production of pesticides [Tuppurainen 2003]."