 Saturday, August 20, 2011 at 12:30PM
Saturday, August 20, 2011 at 12:30PM "It is the main component of most soap-based products, and if you were to look in your bathroom you're 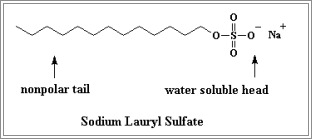 guaranteed to find at least one product containing it, for example, your shampoo or toothpaste [2]. In industry it will be found in engine degreasers or carpet cleaners, for example. It's inexpensive and an excellent foaming agent. It has a high pH as it is an alkali substance and has the appearance of a white powder."
guaranteed to find at least one product containing it, for example, your shampoo or toothpaste [2]. In industry it will be found in engine degreasers or carpet cleaners, for example. It's inexpensive and an excellent foaming agent. It has a high pH as it is an alkali substance and has the appearance of a white powder."
"Sodium lauryl sulfate is a surfactant, which means a molecule that has ampiphilic properties. This means the sulfate head group (shown by the pink shading in the diagram below) is hydrophilic and water soluble, while the 12-carbon-long chain is hydrophobic and water insoluble. It is an anionic surfactant as defined by the sulfate head group, since it has a negative charge. The head group must be sufficiently soluble in water to be classed as a surfactant [2,3]."
Reader Comments