 Thursday, November 29, 2012 at 11:18AM
Thursday, November 29, 2012 at 11:18AM Phospholipids are a class of lipids that are a major component of all cell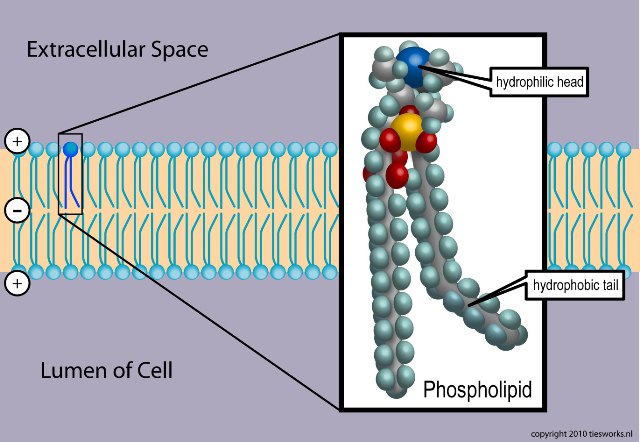 membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from sphingosine instead of glycerol. The first phospholipid identified as such in biological tissues was lecithin, or phosphatidylcholine, in the egg yolk, by Theodore Nicolas Gobley, a French chemist and pharmacist, in 1847. The structure of the phospholipid molecule generally consists of hydrophobic tails and a hydrophilic head. It is usually found with cholesterol molecules which are found in-between the spaces of the phospholipid. Purified Phospholipids are produced commercially by companies like NOF Corporation, VAV Life Sciences, Avanti Polar etc.
membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from sphingosine instead of glycerol. The first phospholipid identified as such in biological tissues was lecithin, or phosphatidylcholine, in the egg yolk, by Theodore Nicolas Gobley, a French chemist and pharmacist, in 1847. The structure of the phospholipid molecule generally consists of hydrophobic tails and a hydrophilic head. It is usually found with cholesterol molecules which are found in-between the spaces of the phospholipid. Purified Phospholipids are produced commercially by companies like NOF Corporation, VAV Life Sciences, Avanti Polar etc.
The 'head' is hydrophilic (attracted to water), while the hydrophobic 'tails' are repelled by water and are forced to aggregate. The hydrophilic head contains the negatively charged phosphate group, and may contain other polar groups. The hydrophobic tail usually consists of long fatty acid hydrocarbon chains. When placed in water, phospholipids form a variety of structures depending on the specific properties of the phospholipid. These specific properties allow phospholipids to play an important role in the phospholipid bilayer. In biological systems, the phospholipids often occur with other molecules (e.g., proteins, glycolipids, cholesterol) in a bilayer such as a cell membrane.[1] Lipid bilayers occur when hydrophobic tails line up against one another, forming a membrane hydrophilic heads on both sides facing the water.
Reader Comments