 Wednesday, August 29, 2012 at 11:07AM
Wednesday, August 29, 2012 at 11:07AM "In particle physics, a gauge boson is a force carrier, a bosonic particle that carries any of the fundamental interactions of nature.[1][2] Elementary particles, whose interactions are described by a gauge theory, interact with each other by the exchange of gauge bosons—usually as virtual particles."
any of the fundamental interactions of nature.[1][2] Elementary particles, whose interactions are described by a gauge theory, interact with each other by the exchange of gauge bosons—usually as virtual particles."
"The Standard Model of particle physics recognizes three kinds of gauge bosons: Photons, which carry the electromagnetic interaction; W and Z bosons, which carry the weak interaction; andgluons, which carry the strong interaction."
"Isolated gluons do not occur at low energies because they are color-charged, and subject to color confinement."
"In a quantized gauge theory, gauge bosons are quanta of the gauge fields. Consequently, there are as many gauge bosons as there are generators of the gauge field. In quantum electrodynamics, the gauge group is U(1); in this simple case, there is only one gauge boson. In quantum chromodynamics, the more complicated group SU(3) has eight generators, corresponding to the eight gluons. The three W and Z bosons correspond (roughly) to the three generators of SU(2) in GWS theory."






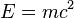 ) of Einstein and the existence of the
) of Einstein and the existence of the 


