Monday, February 25, 2013 at 11:01AM
Monday, February 25, 2013 at 11:01AM "Estradiol (E2 or 17β-estradiol, also oestradiol) is a sex hormone. Estradiol is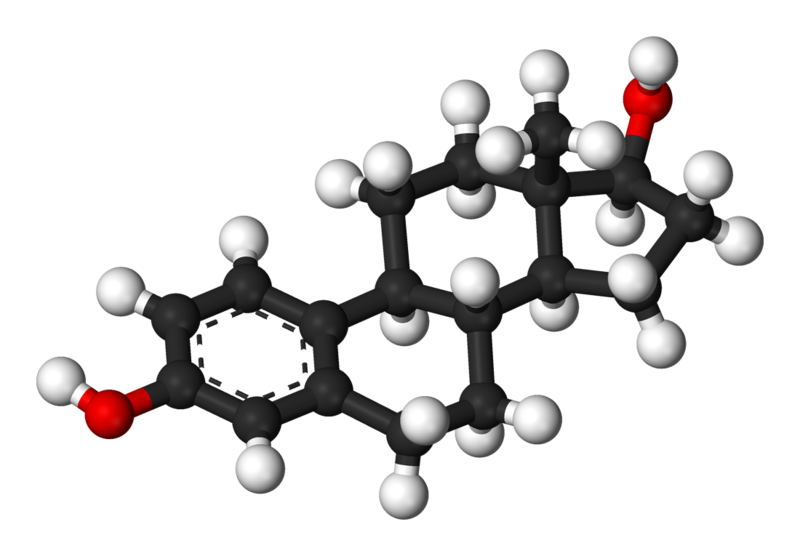 abbreviated E2 as it has two hydroxyl groups in its molecular structure. Estrone has one (E1) and estriol has three (E3). Estradiol is about 10 times as potent as estrone and about 80 times as potent as estriol in its estrogenic effect. Except during the early follicular phase of the menstrual cycle, its serum levels are somewhat higher than that of estrone during the reproductive years of the human female. Thus it is the predominant estrogen during reproductive years both in terms of absolute serum levels as well as in terms of estrogenic activity. During menopause, estrone is the predominant circulating estrogen and during pregnancy estriol is the predominant circulating estrogen in terms of serum levels. Estradiol is also present in males, being produced as an active metabolic product of testosterone. The serum levels of estradiol in males (14 - 55 pg/mL) are roughly comparable to those of postmenopausal women (< 35 pg/mL). Estradiol in vivo is interconvertible with estrone; estradiol to estrone conversion being favored. Estradiol has not only a critical impact on reproductive and sexual functioning, but also affects other organs, including the bones."
abbreviated E2 as it has two hydroxyl groups in its molecular structure. Estrone has one (E1) and estriol has three (E3). Estradiol is about 10 times as potent as estrone and about 80 times as potent as estriol in its estrogenic effect. Except during the early follicular phase of the menstrual cycle, its serum levels are somewhat higher than that of estrone during the reproductive years of the human female. Thus it is the predominant estrogen during reproductive years both in terms of absolute serum levels as well as in terms of estrogenic activity. During menopause, estrone is the predominant circulating estrogen and during pregnancy estriol is the predominant circulating estrogen in terms of serum levels. Estradiol is also present in males, being produced as an active metabolic product of testosterone. The serum levels of estradiol in males (14 - 55 pg/mL) are roughly comparable to those of postmenopausal women (< 35 pg/mL). Estradiol in vivo is interconvertible with estrone; estradiol to estrone conversion being favored. Estradiol has not only a critical impact on reproductive and sexual functioning, but also affects other organs, including the bones."