Wednesday, September 21, 2011 at 10:40AM
Wednesday, September 21, 2011 at 10:40AM "Glucose is a simple carbohydrate, which means it contains carbon, hydrogen and oxygen. Sugars like glucose 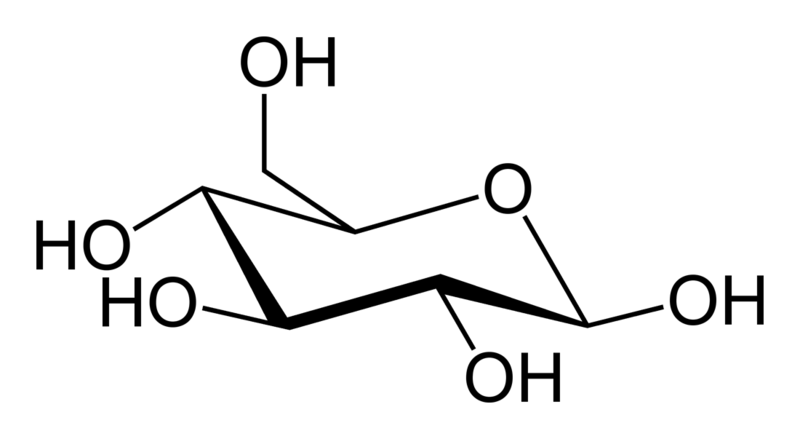 (C6H12O6) with six carbon atoms are referred to as hexoses, and it has one sugar unit so it is a monosaccharide. Its name comes from the Greek glykos, which means 'sweet'."
(C6H12O6) with six carbon atoms are referred to as hexoses, and it has one sugar unit so it is a monosaccharide. Its name comes from the Greek glykos, which means 'sweet'."
"In 1888 one of the world's most important chemists, Emil Fischer, discovered the three sugars, glucose, fructose and mannose. By 1890 he was the first chemist to synthesize all three of these sugars starting from glycerol. He was awarded the 1902 Nobel prize in Chemistry."