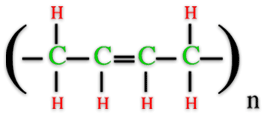
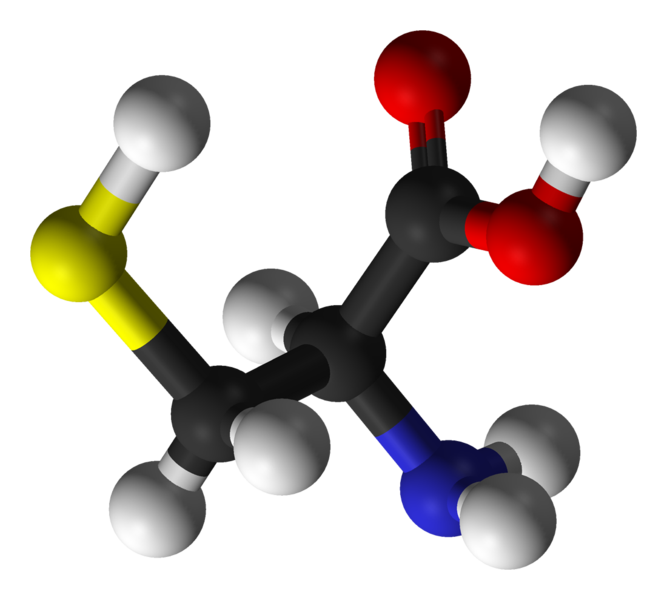
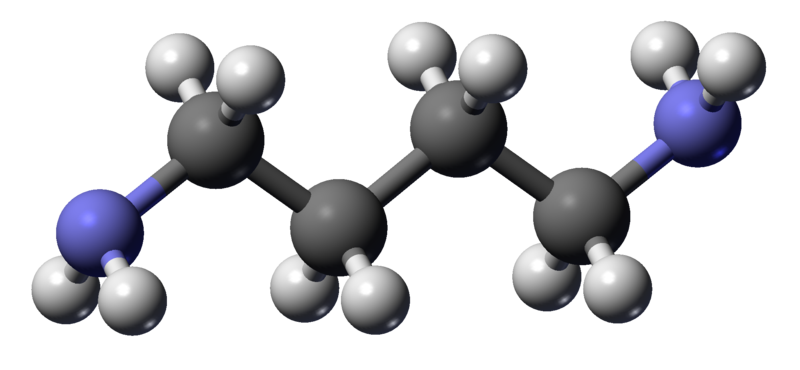
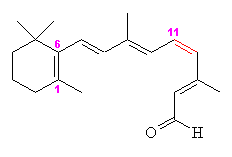
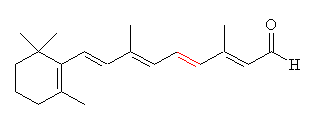
Thursday, October 20, 2011 at 11:29AM
Thursday, October 20, 2011 at 11:29AM "A chlorofluorocarbon (CFC) is an organic compound that contains carbon, chlorine, and fluorine, produced 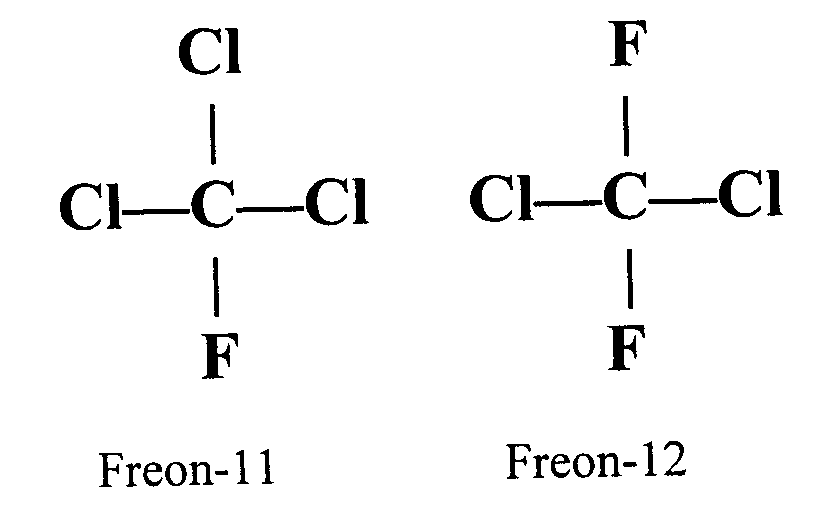 as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons (HCFCs), which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon. The most common representative is dichlorodifluoromethane (R-12 or Freon-12). Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), and solvents. The manufacture of such compounds is being phased out by the Montreal Protocol because they contribute to ozone depletion."
as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons (HCFCs), which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon. The most common representative is dichlorodifluoromethane (R-12 or Freon-12). Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), and solvents. The manufacture of such compounds is being phased out by the Montreal Protocol because they contribute to ozone depletion."